Good podcast? Give it some love!
Hogan Lovells
Talking the Cure by Hogan Lovells
A Business podcast
Good podcast? Give it some love!
Rate Podcast
Episodes of Talking the Cure by Hogan Lovells
Mark All
Search Episodes...
In this episode of "Pharma: The Last Word," Phil Katz and Hein van den Bos discuss early access programs in the EU, exploring the various types of programs, their practical implications, and the regulatory challenges. They delve into compassion
In this episode of "Pharma - The last word," host Phil Katz, discusses regulatory and legal trends in the pharma and biotech industries with Komal Karnik Nigam. They delve into the FDA's evolving approach to data packages required for drug appr
Welcome to the inaugural episode of "Pharma: The last word," a podcast hosted by Partner Phil Katz from Hogan Lovells. This series aims to dive into the intricate world of pharmaceuticals and biotech regulatory and legal landscapes, exploring n
In this episode of Talking the Cure Hogan Lovells Life Sciences and Health Care Podcast Ruud van der Velden and Chantal Van Dam highlight the importance of protecting intellectual property and trade secrets in the life sciences and health care
In this episode Jane Summerfield and Owen Robinson discuss UK medical device regulation, focusing on the regulatory roadmap published by the MHRA, its implications for businesses, and the process for legislation development. They highlight the
In the second part of the episode, Arne Thiermann is leading the conversation with Richard Backhaus, and Lars Cornels discussing the integration and implications of digital health, artificial intelligence, and legal considerations i
In this first part of the episode, Arne Thiermann is leading the conversation with Richard Backhaus, and Lars Cornels discussing the integration and implications of digital health, artificial intelligence, and legal considerations in the medica
In this episode, Robert F. Church takes the time to look back on his career with us. We talk about what ultimately led him to become a lawyer, how he ended up at Hogan Lovells, and how he became the institution in clinical trials. Of course, w
In this episode, Alice Valder Curran sits down with us to talk about her impressive career, why she ultimately chose Hogan Lovells as her employer, and what it takes to be successful in this area of the industry, even in times when you can only
In this episode, Randy Prebular sits down with us and we talk about his impressive professional career, how he actually wanted to be a pediatrician at the beginning of his career, and how he then went from being a research scientist to a partne
In this podcast episode, Alice Curran and Cullen Taylor discuss the implications of the Inflation Reduction Act (IRA) on the life sciences and healthcare sector. They explore how IRA can impact drug pricing, development, collaboration, and part
In this episode оf 'Talking the Cure,' we dive into the latest developments іn clinical trials diversity, driven by the Food and Drug Omnibus Reform Act (FDORA). Join Stephanie Agu, Akosua Tuffuor and Deborah Cho as we explore FDORA's key
Arne Thiermann and Nicole Saurin talk about the current state and development of artificial intelligence in the medical device industry. How will AI medical devices be classified by the AIA Proposal? What is the relationship between the AIA Pro
In this episode, Beth L. Roberts (Partner, Washington, D.C.) is talking to Kwame Ulmer (Founder and Executive Director) about MedTech Color, its mission, programs, and resources they provide. In addition, they’re discussing which hot topics in
In this episode, Ina Brock invites us to her home and tells us that she actually wanted to become a journalist, but chance brought her to law. And the result of this decision is an impressive career, which we review together, Ina gives us insig
Robert F. Church, Sarah Thompson Schick, Stephanie Agu, and Akosua Tuffuor, J.D. discuss recent developments in FDA’s and industry’s approaches towards clinical trials and ensuring meaningful trial participation from members of historically und
What to expect if you want to repurpose existing UK real estate, in particular, offices/retail to laboratory space? Graham Cutts will give an overview of potential building reconfigurations, occupier profiles, and the current industry hot topic
What’s on the horizons for orthopedic devices? In this episode, Michael Kasser and Jemin Jay Dedania talk about their journey in the field of orthopedic devices, why orthopedics is still a little like the wild west of the FDA, how new technolog
In this episode, Andreas von Falck will tell us that he actually wanted to work for an NGO, but then followed his father’s path and became one of the most successful patent attorneys in Germany. Andreas will tell us how he came to his Life Scie
In this episode, Dr. Victor Stephani, Chief Of Staff at the German digital health high flyer HelloBetter, and Arne Thiermann, a life sciences partner in the Hamburg office and very familiar with the regulations of digital health applications, d
In the first episodes of Season 3, Jonathan S. Kahan, partner and passionate lawyer in the Medical Device and Technology Regulatory practice, took the time to talk to our host, Julius Bülow, about his work as a lawyer, how he built his practice
In this episode, I talk with Dr. Tina Welter-Birk and Komal Karnik Nigam about the Early Access Programs (EAPs), known as Expanded Access Programs in the US, in Germany, and the United States, which allow pre-approval access to medicines for ce
In this episode, Christian Di Mauro, who is heading the Italian litigation and arbitration practice talk about his 17 years of experience working with the Life Sciences and Health Care industry and being involved in products including pharmaceu
In this episode, Thierry Meillat, Stefan Martin, Eckard Schwarz, and Zachary Siegel talked about the various return to work policies, testing at work sites, mask mandates, and vaccinations. Due to the fast developments of the situation, a few c
In this episode, Lowell M. Zeta, who just rejoined the firm after serving as Senior Counselor to the Commissioner at FDA, is going to talk to Philip Katz about his work on cross-cutting and high-priority initiatives promoting innovation and add
Join Podchaser to...
- Rate podcasts and episodes
- Follow podcasts and creators
- Create podcast and episode lists
- & much more
Unlock more with Podchaser Pro
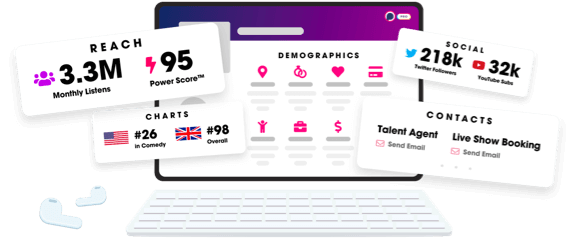
- Audience Insights
- Contact Information
- Demographics
- Charts
- Sponsor History
- and More!
- Account
- Register
- Log In
- Find Friends
- Resources
- Help Center
- Blog
- API
Podchaser is the ultimate destination for podcast data, search, and discovery. Learn More
- © 2024 Podchaser, Inc.
- Privacy Policy
- Terms of Service
- Contact Us
